Toward Precision Psychiatry: Innovations and Prospects in Treating Depression
Daphna Laifenfeld, PhD; Claudia Albeldas, MSc; Talia Cohen Solal, PhD; Roger S. McIntyre, MD, FRCPC; Stephen Stahl, MD PhD


Abstract
Importance: Major depressive disorder is a heterogeneous disorder affecting over 280 million people globally. Despite multiple treatment options, individual response to drugs varies significantly, and most patients go through a trial-and-error approach, resulting in multiple drug iterations before alleviation of symptoms is achieved. Treatment optimization is further complicated by lack of full elucidation of the neurobiology of depression. The high prevalence of nonresponse, coupled with the detrimental effects of prolonged disease on patient welfare, economic burden, and increased likelihood of recurrence, substantiates the critical need for robust tools capable of precisely matching patients with their most effective and safe treatment options in a time-sensitive manner.
Observations: Research into technologies that tailor treatments to individual patients based on their unique molecular and cellular characteristics has led to the development of precision medicine tools ranging from pharmacogenetics through peripheral biomarkers and neuroimaging to a platform that uses patient-derived neurons as a substrate for in vitro patient-specific functional readouts.
Conclusions and relevance: Novel precision medicine tools in depression are being introduced that aim to identify the optimal treatment for each patient. Such tools have the potential to significantly improve depression management by guiding treatment selection for prescribers and people with lived experience.
Author affiliations are listed at the end of this article.
Major depressive disorder (MDD) confers an enormous global societal and economic burden. The prevalence of MDD significantly increased with the COVID-19 pandemic, stemming from a combination of social isolation and the aftermath of the illness itself.1,2 While multiple treatments for alleviating depression symptoms exist, including more than 2 dozen approved antidepressants, psychotherapy, and noninvasive neurostimulation, disease management is confounded by patient heterogeneity and a lack of treatment-guiding tools. Advances in precision medicine have the potential to revolutionize the management of depression while reducing the disease burden.
MDD is one of the most severe and common mood disorders, affecting in excess of 280 million people globally and causing over 700,000 depression-related deaths annually.3 Characterized by persistent sadness, loss of interest or pleasure, low energy, reduced appetite, disturbed sleep patterns, and disrupted daily activities and psychosocial functions, this widespread and severely disabling disorder is associated with impaired daily functioning, diminished quality of life, and increased mortality and health care utilization.4–7
Patients diagnosed with MDD often experience recurring episodes, with studies showing a recurrence rate of over 50%. Each subsequent episode increases the likelihood of further episodes.8–11 Despite the availability of numerous approved drugs, a significant proportion of patients fail to respond adequately to the first-line treatment, inviting the need for multiple often unsuccessful treatment trials.12 There is a significant gap between the existence of multiple approved medications to treat depression and the lack of tools and guidelines to support the physician in choosing the optimal medication for their patient.
The emergence of novel mechanisms for pharmacologic intervention in depression, the imminent need to move away from trial-and-error prescription practices, and the advent of technology have resulted in new tools in depression disease management. The goal of this review is to highlight the most recent advances and technologies supporting new clinical algorithms for depression treatment, which are in various stages of market introduction.
CURRENT THERAPY OPTIONS FOR MDD
Treatment of MDD aims to achieve remission that is maintained over time in each patient. The early antidepressant medications, monoamine oxidase inhibitors (MAOIs) and tricyclic antidepressants (TCAs), were discovered largely serendipitously.13–17 While having potent antidepressant effects, the lack of target specificity of the MAOIs and TCAs leads to significant side effects, rendering them less tolerable by patients. The finding that MAOIs and TCAs decrease symptoms of depression supported the monoamine theory of depression, and subsequently, selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) were developed to treat depression. Overall, due to their potency, combined with improved tolerability, the SSRIs and SNRIs are the most widely prescribed medications and are considered the first line of treatment.18 Recently, rapid-onset antidepressants, like intranasal esketamine12 and psychedelics (eg, psilocybin, lysergic acid diethylamide, and ayahuasca-dimethyltryptamine19), have begun to show great promise, especially for treatment-resistant depression, by enhancing neuroplasticity through mechanisms involving serotonin and glutamate pathways.20–23 The development of novel antidepressants with rapid and sustained effects could represent a significant breakthrough in the treatment of MDD.24,25 Still, more robust trials are needed to gain an in-depth understanding of their mechanisms and long-term impacts,26 and caution should be exercised given the potential risk of recreational abuse.27–29 At the time of writing this review, however, psychedelics were not considered first-line medications. Finally, nonpharmacologic approaches, such as electroconvulsive therapy, repetitive transcranial magnetic stimulation (rTMS), and theta burst stimulation,30 also effectively modulate cortical excitability and plasticity, offering alternatives for resistant cases.
OPTIMIZING TREATMENT CHOICE FOR EACH MDD PATIENT
Despite the abundance of antidepressant medications available, a significant number of patients either fail to respond to the different treatment options or experience intolerable side effects from medications.31,32 Unremitted illness is associated with a worsened long-term prognosis, increased side effect burden, and exacerbation of other medical illnesses.33–35 Most of the nonresponse to medications is likely due to the heterogeneity of MDD in terms of its pathophysiology, etiology, and underlying neurobiological mechanisms, underscoring the critical need for the development of robust tools capable of precisely matching patients with the treatment options most effective for their biology.
Precision Medicine: The Current Landscape in Depressive Disorders
Precision medicine aims to tailor healthcare, and drug treatment in particular, to the individual characteristics of each patient. The past 2 decades have witnessed a rise in precision medicine approaches in both drug development and disease management. In particular, the field of oncology has been revolutionized by precision approaches, from molecular diagnostics to targeted treatments, enabling breakthroughs in disease management for oncology patients.36 Thus, the first approval of a precision medicine was Herceptin (trastuzumab) in 1998 for human epidermal growth factor receptor 2–positive breast cancer, followed by a plethora of personalized medicine treatments for various cancers and accompanying diagnostic tools. The field of mental health has lagged behind oncology, with precision medicine approaches confined to pharmacogenetic consideration. This is largely driven by the lack of access to the target tissue, in addition to mechanisms of disease and drug action that are not fully elucidated. The past 15 years have seen breakthroughs in this domain that have included multiple biochemical, cellular, and physiologic strategies, ranging from markers of individual drug metabolism designed to identify potential dosing effects or unwanted side effects37–39 and patient neuroimaging at different time resolutions40–42 to clinical data machine learning (ML)–driven methods without direct sampling43–46 and cell culture methods.47 Table 1 summarizes approaches to mental health disorders being developed or already available for commercial use.
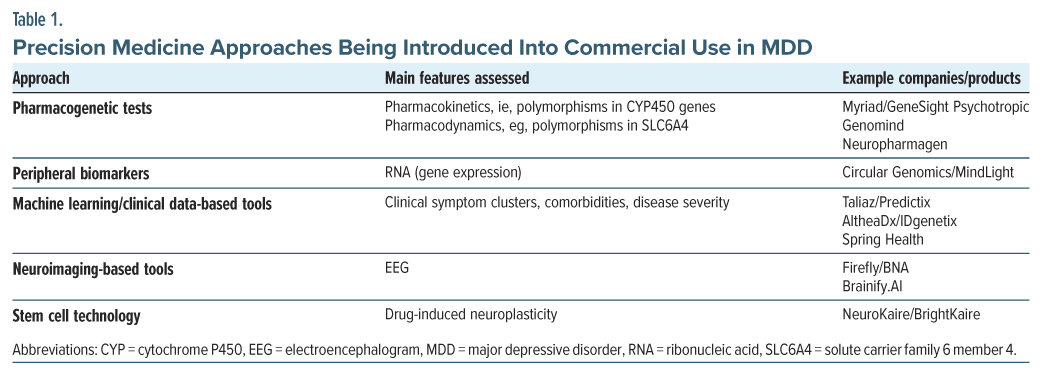
Pharmacogenetic Tests
Over 20 tests are now available commercially that are designed to utilize pharmacogenetic signals for clinical insight into antidepressant suitability and are listed in the National Center for Biotechnology Information Genetic Testing Registry.48 Some of the most well-known include GeneSight and Genomind. They test a plethora of genes implicated in drug-gene interactions, assembled through information from databases including https://www.pharmgkb.org and https://cpicpgx.org. The majority of covered genes and associated variants are the cytochrome P450 (CYP) genes, encoding the CYP enzyme. Variants in these genes, including CYP2B6, CYP2C19, CYP2C9, CYP2D6, CYP3A4, and CYP3A5, lead to altered metabolism of a variety of medications, including several antidepressants, resulting in increased or decreased effective dose and exacerbated drug-drug interactions.38,49 Additional genes, included in some pharmacogenetic tests, include those that may be associated with drug response, such as the SLC6A4 (solute carrier family 6 member 4) gene, for which low expression is correlated with lower SSRI efficacy. Finally, tests may examine variations correlated with side effects, such as polymorphisms in the 5-HT2A (5-hydroxytryptamine 2A) receptor gene, which are correlated with increased side effects in paroxetine and potentially other SSRIs.37,40 One of the largest studies providing clinical validation for the use of pharmacogenetic measures was the GUIDED study, which used a combinatorial approach consisting of a weighted algorithmic assessment of multiple pharmacogenetic and pharmacodynamic genes to predict patient response.50 At 8 weeks, the 1,100-participant study found significant improvements in response and remission, measured by Hamilton Depression Rating Scale scores, in patients whose treatment was guided by PGx. The results of subsequent studies yielded inconsistent results, with some showing significant improvements and others only nonsignificant trends.51,52
The position statement of the American Psychiatric Association (APA) Council of Research Workgroup on Biomarkers and Novel Treatments reviewed recent scientific literature published between 2017 and 2022 regarding the application of pharmacogenetic tools in treatment selection for MDD. Considering primary end point failures and inherent methodological limitations observed in the reviewed studies, the analysis concluded that these newer studies do not provide enough evidence to change the recommendations set forth in the 2018 APA report. Consequently, the use of commercially available pharmacogenetic tools to guide treatment decisions in MDD remains unsupported.53
ML Approaches for Clinical Data-Based Tools
The advent of artificial intelligence (AI) and ML methodologies has enabled analysis of complex relationships between features, allowing the detection of high-dimensional interactions beyond what a human can process. Leveraging such methodologies, clinical data-driven approaches have attempted to use patients’ clinical and demographic data to develop predictive models based on relevant underlying features for optimal antidepressant response. For example, Chekroud et al54 used the landmark Sequenced Treatment Alternatives to Relieve Depression (STAR*D) dataset to develop a prediction algorithm for the probability of drug response and validated their prediction accuracy in the independent dataset from the Combining Medications to Enhance Depression Outcomes trial. They were able to reach between 51.4% and 59.7% treatment accuracy depending on the medications tested. The effectiveness of using symptom clusters specifically was also addressed and was demonstrated to associate with differences in efficacy between treatments.54 This technology has been adopted by Spring Health as a component of their employer offering to improve patient outcomes in the work setting.
In addition to these 2 approaches, ML techniques have aimed to integrate clinical and pharmacogenetic data types within the same platform to improve prediction of antidepressant response. Taliaz et al55 used the STAR*D dataset in addition to data from the Pharmacogenomics Research Network Antidepressant Medication Pharmacogenomic Study (PGRN-AMPS) to develop and test algorithms based on pharmacogenetics combined with clinical data-driven approaches. This retrospective analysis used a purpose-trained algorithm for responses to 3 antidepressants (SSRI and SNRI) and found an average balanced accuracy of 70%, using the Quick Inventory of Depressive Symptomatology–Self-Report questionnaire as an assessment measure, when combined with PGx readouts. Similarly, Bradley et al56 used AltheaDx’s tool, which combines variants from 10 genes with data on patient concomitant medications, to make treatment recommendations in a randomized blinded study of 685 patients suffering from depression and/or anxiety. The study demonstrated significantly higher response and remission rates in the depression patients in the group guided by pharmacogenetics and concomitant medication data versus the unguided control arm.
Neuroimaging-Based Methods
Personalized neuroimaging methods for predicting antidepressant effect have arisen as an alternative to pharmacogenetics for antidepressant treatment prediction. For example, Nguyen et al57 utilized functional magnetic resonance imaging (fMRI) data collected from the Establishing Moderators and Biosignatures of Antidepressant Response in Clinical Care (EMBARC) study. The algorithm combined fMRI data with clinical and demographic measures. Their model achieved R2 of 28% for placebo, 34% for bupropion, and 48% for sertraline and highlighted activity in areas including prefrontal, orbitofrontal, and cingulate, but interestingly also caudate and cerebellum.
Similarly, using a resting-state electrophysiologic paradigm also collected as part of the EMBARC study, Rolle et al58 conducted a non-prespecified secondary analysis examining the potential predictive effects of electrophysiologic measures, including power envelope connectivity of standard frequency ranges and node strength among regions of interest. This analysis found that increased α and lower γ connectivity, particularly in parietal regions, was predictive of better response (measured by the Hamilton Rating Scale for Depression) to placebo and worse response to antidepressant treatment. The observation of local slowing is consistent with a model of thalamocortical dysrhythmias based on differential Ca2+ channel expression and posited to underlie a range of neurological and psychiatric disorders.59 However, it is notable that while both anhedonia and severity were significantly associated with resting-state electroencephalogram (EEG) signals, these results explained only a small (5%–10%) level of connectivity variance. Building on this using resting-state EEG, as well as task- and stimulation (TMS)-driven responses, Wu et al60 examined EMBARC data using a ML algorithm tuned to prediction of antidepressant response. The study found a predictive signature (open-eye α band activity) for 1 SSRI (sertraline) that was also able to distinguish between response to the sertraline versus placebo arm. These new technologies, in which brain function measures combined with AI enable response prediction, are being used by Alto Neurosciences for stratifying patients to their optimal pipeline candidate.
Stem Cell–Based Technology
In the past decade, the ability to induce cell pluripotency as a means to derive specific cell types has led to prodigious advances in research into human disease, as well as in potential medical applications. An exciting and novel opportunity is thus introduced for studying previously inaccessible cells, including neuronal cells in individual patients. Two groups have demonstrated the potential of leveraging this technology to generate individual patient neurons from human-induced pluripotent stem cells (hiPSCs) for treatment prediction in depression. Vadodaria et al61 generated serotonergic neurons from patients who had participated in the PGRN-AMPS study using hiPSC technology and revealed that neurons derived from the nonresponder patients showed altered neurite growth and morphology compared to healthy controls and responders.
A second group, Avior et al,62 leveraged this technology to generate frontal cortical neurons from banked samples from the STAR*D cohort. The effect of antidepressants on these neurons was then measured, focusing on neuronal plasticity and its restoration as part of antidepressant mechanism of action. Notable differences in drug-induced features of neuroplasticity such as spine maturity and synaptic colocalization were observed in neurons derived from drug responders versus nonresponders. RNA sequencing data provided further mechanistic confirmation that patient-derived neurons reflect clinically relevant antidepressant effects, suggesting that readouts from these neurons can be used to quantify drug-induced neuroplasticity associated with clinical drug response. AI and ML methodologies are applied to integrate the features into a robust neuroplasticity score as part of a commercial tool to support treatment choice optimization by GenetikaPlus/NeuroKaire.63
CONCLUSION
MDD is a heterogeneous disorder that encompasses a variety of clinical conditions associated with specific biological imbalances, which lead to different response patterns to various classes of antidepressants.64 Despite an abundance of available antidepressant treatment options, most patients do not respond to first-line medications, and approximately a third of patients undergo more than 2 treatment rounds before identification of their optimal medication.65,66 Even after successful treatment, up to 85% of MDD patients experience a relapse within 10 years of their first episode.67 Over the past decade, several tools aiming to address the issue of patient heterogeneity in response to antidepressant treatment have been introduced. These tools include both pure pharmacogenetic tools and combinatorial decision support tools, which have been and are being integrated into clinical practice. The limited but increasing use of such tools in clinical practice demonstrates both the necessity and the willingness of physicians to use tools to guide their decision-making regarding antidepressant drug prescription.68 State-of-the-art technologies in this domain attempt to circumvent the lack of access to the brain, the target organ for antidepressant effects, either through neuroimaging or through patient-derived neurons, constituting a patient-specific “brain-in-a-dish” model.62 With new technologies making their way into clinical practice, a new era of personalized medicine in the treatment of depression is anticipated, potentially alleviating a substantial proportion of the disease burden.
Clinical Points
- The majority of depressed patients go through a trial-and-error approach, resulting in multiple drug iterations before the optimal drug is identified and alleviation of symptoms is achieved.
- New precision medicine tools are being introduced to the market to enable identification of the right drug for each patient.
- The clinical utility of these tools is dependent on their performance as well as on their ease of use for the physician and patients.
Contact Us
78 John Miller Way,
Kearny, NJ 07032, USA
Yigal Alon Street 126
Tel Aviv-Yafo, Israel
